- Jonathan Palmer
- 3 years ago
- Views:
Transcription
Resources Here to. Evalutating the contribution of the column design to the ion exchange performance by calculation of. At puredesign@purolite.com, for the. Ion Exchange Design Hand calculation. Brian Windsor (Purolite International Ltd) Ion Exchange Design Hand calculation. Program Report No. 124 ION EXCHANGE.
1 Ion Exchange Design Hand calculation Brian Windsor (Purolite International Ltd)
2 Introduction Before design programmes were introduced, every engineer had to calculate the design by hand using resin manufacturers data. Each engineer had their own way to carry out in their calculation. Before starting the design ideally you need to know some basic information: 1. Maximum / Average / Minimum flow rate and roughly how many hours per day the maximum flow rate is required (m 3 /h). 2. Daily requirement (m 3 /day) over how many hours. 3. Design water analysis 4. Cation regenerant (sulphuric or hydrochloric acid)
3 Flow rate 1. If the maximum demand is for a short period, or if there is a wide range of operating flow rates, then you can design the plant on the average demand and include a larger treated water storage to cater for the maximum flow rate or variations in demand. 2. It is always to keep the plant in operation rather than operating on an on-off basis with lots of stopping and starting. 3. Resins can operate over a range of flow rates, but the design of ion exchange columns is often very basic and many cannot accommodate very low flow rates. Poor distribution / collection is often encountered at low flow rates leading to channelling and poor performance.
4 Cycle Time / No of Streams The cycle time for each cation anion pairing is determined by the water analysis, flow rate and number of streams. Where demineralised water is critical to the sites operation then normally the client will require a standby stream to cover for regeneration time of the other stream(s) and allow some basic maintenance. Most commonly encountered plants are therefore 2 x 100% or 3 x 50% duty, but where very high flow rates are encountered 4 x 33% duty and 5 x 25% duty streams have been supplied. A cation anion pair can be regenerated in under 2 hours if simultaneous regeneration is employed and with short cycle plant it is even quicker. Mixed beds are more complicated, but are regenerated less frequently and usually take 2 to 3 hours.
5 Cycle Time / No of Streams Ignoring short cycle plants, then classical designs are often base on 8 or 12 hours on line between regenerations depending on the water analysis used for the design. Longer cycle times are encountered when the raw water TDS is low. Such thin waters often see plants designed on 24 hours on line or even longer. We will now select the basis for our design calculations.
6 Design Analysis 1. Knowledge of the maximum and typical analysis is critical when choosing the cycle time. No point choosing short cycle time on the design analysis if the worst water has a much higher TDS. This will mean the time between regenerations is too short. 2. The analysis should balance i.e. The cations and anions expressed as mg/l CaCO 3 or in meq/l should be very similar (within 5%) 3. You have to design the plant to cope with the worst water, but if that water is very infrequently seen then all design operating costs and design decisions need to be based on the typical water analysis.
7 Maximum Water Analysis (Worst Water) If designed on the worst water analysis presented by the end user the water may not balance as the client may have cherry picked the highest recorded level for each ion. These highest level for all ions will not occur on the same day and hence it will not balance. Then you can round down the highest category to give the balanced analysis as naturally occurring waters must balance. In our calculation the end user has given us the analysis of his anticipated worst water.
8 Design Basis for this Calculation Flow Rate 60 m3/h (for 24 hours per day) Important application so standby stream required 2 x 100% duty. Hydrochloric acid for cation regeneration. Water temp 10 centigrade. Worst Water Analysis from client (all expressed in mg/l as CaCO 3.) Cations Anions Calcium (Ca) 110 Bicarbonate (HCO 3 ) 100 Magnesium (Mg) 55 Sulphate (SO 4 ) 50 Sodium (Na) 100 Chloride (Cl) 75 Potassium (K) 11 Nitrate (NO 3 ) 25 TOTAL CATIONS 276 TOTAL ANIONS 250 Greater than 10% difference!
9 Design Basis Corrected Analysis Corrected Worst Water Analysis to balance (all expressed in mg/l as CaCO 3) ) Cations Anions Calcium (Ca) 100 Bicarbonate (HCO 3 ) 100 Magnesium (Mg) 50 Sulphate (SO 4 ) 50 Sodium (Na) 90 Chloride (Cl) 75 Potassium (K) 10 Nitrate (NO 3 ) 25 TOTAL CATIONS 250 TOTAL ANIONS 250 Design analysis to go forward In addition we will assume a Reactive Silica of 5 mg/l as CaCO 3. It is a ground water and contains negligible dissolved organics.
10 Degassing tower The inclusion of a degassing tower to remove the bicarbonate after the cation, when it is converted to carbonic acid, has to be decided before the design is commenced. If the bicarbonate level is above 40 mg/l as CaCO 3 then it is normally cost effective to include a degasser, this is particularly the case on engineered design where a neutral effluent is often required from the waste water produced and plant costs are greater. However, on small, low flow rate, standard plants many companies do not supply a degasser as the capital cost associated with the tower, degassed water pumps (stainless steel) makes the pay back period less attractive and here all the bicarbonate load is often removed by the anion stage. On this design with 150 mg/l bicarbonate we will have a degasser tower.
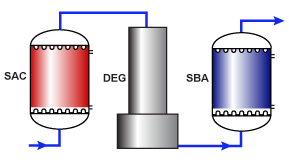
11 Design Approach In all cases however you have to design from the back of the plant. If you have a cation anion mixed bed system then you start with mixed bed calculation, then anion and then the cation. This way you can calculate or estimate the waste water required which must pass through the preceding unit during the design. For this example I will assume a simple co-flow regenerated system of cation anion followed by a polishing mixed bed. Counter flow regeneration is a little more complicated as you have to estimate the additional treated water required for regenerant injection and slow rinses on the cation and anion beds.
12 Polishing Mixed Bed - Design basis After SAC / SBA resins the loading on to polishing mixed beds is very low and so these units are sized on flow rate which makes it very simple. The basis I use is: Vessel sizing based on specific velocity of 60 m 3 /m 2 /h. 600 mm minimum bed depth for cation / anion components. Anion regeneration level 65 g/l with cation regen level designed to give substantially self neutralising effluent. Depending on cation load, approx. 62 g/l for HCl and 80 g/l for sulphuric acid gives a neutral effluent. If not worried about neutral effluent then regen levels as low as 60 g/l NaOH and 48 g/l HCl or H 2 SO 4 have been used.
13 Polishing Mixed Bed - Sizing Applying these parameters to 60 m 3 /h flow rate then: 60 m 3 /h divided by 60 m/h = Minimum area 1 m 2 required. Hence based on UK vessel sizing = 1219 mm diameter (1.16 m 2 ) Resin volume per unit = 0.6 m x 1.16 m 2 = m3 (696 litres) Rounded up to the nearest 25 litres = 700 litres of each component. Caustic applied at 65g/l regen level = 65g/l x 700 / 1000 = 45.5 Kg (as 100% NaOH). HCl applied at 63 g/l regen level = 63 g/l x 700 / 1000 = 44.1 Kg (as 100% HCl). (Metric Sizing 1200 mm diameter with 675 litres of each resin)
14 Polishing Mixed Bed - Operation Mixed beds should never be run near to exhaustion due to the long run length this has little effect on plant operating costs. Historically I tend to use the following guide capacities to determine run length of a polishing mixed bed based on the cation / anion leakage. Anion reactive silica loading should not exceed 9 g/l (6 g/l used by some) Cation sodium loading should not exceed 15 g/l. These are highly conservative. Hence good quality obtained at all times!
15 Anion Design Anion analysis after Degasser Tower After the degassing tower the bicarbonate anion loading will be reduced typically to < 5 mg/l CO 2 as CaCO 3 Therefore the anion load based on the original raw water will now be: Anions Sulphate (SO 4 ) 50 Chloride (Cl) 75 Nitrate (NO 3 ) 25 Reactive Silica 5 Carbon Dioxide 5 Anion Load = 160 mg/l as CaCO 3
16 Anion Design Gross water production per cycle Early we chose 8 hour on line for the worst water. Therefore volume of water treated per cycle would be: (8 hours x 60 m) + MB regen water (Note MB normally uses between 12 and 20 BV of water per regen.) We will use 15 BV for the calculation, so with 1400 litres of resin per mixed bed it needs 15 x 1400 = litres (21 m 3 ) Therefore anion volume of treated water = (8 x 60) + 21 m 3 = 501 m 3
17 Anion Design Anion load per cycle Therefore anion volume of treated water = (8 x 60) + 21 m 3 = 501 m 3 The anion ionic load per cycle is therefore 501 m 3 x 160 mg/l / 1000 = Kg as CaCO 3 Now we have the load per cycle to calculate the resin volume we need to now calculate from the resin manufacturers data the working capacity of the resin. For the basis of this calculation, with a low organics content I am basing on a gel, polystyrenic, anion resin with a high capacity. This type of product is available from all the leading suppliers.
18 Anion Design Capacity Correction Factors For a type 2, gel, polystyrenic, anion resin the working capacity is determined by a base capacity dependent on regen level (amount of caustic applied. This capacity then has various correction factors applied and each manufacturers graphs presented differently. The percentage sulphate in the anion load. The percentage CO2 in the anion load. The silica endpoint for regeneration. The bed depth (if shallow below 0.7 m). We can ignore this with our design as with the size of plant our bed depth will be between 1 and 1.5 m. The percentage silica in the anion load and regenerant temperature. Co-flow plant regen levels tend to be between 55 and 80 g/l. However, regen levels are sometimes encountered outside this range.
19 Anion Design Base Resin Capacity For this co-flow regenerated design I have chosen a regeneration level of 60 g/l NaOH. From the resin engineering bulletin this gives a base working capacity of 0.75 eq/l. This is 37.5 g/l as CaCO 3 (0.75 x 50)
20 Anion Design Anion Capacity Adjustment Sulphate percentage in anion load 50 mg/l / 160 mg/l x 100 = 31.25%. From graph correction factor = 0.95 Carbon Dioxide percentage in anion load 5 mg/l / 160 mg/l x 100 = 3.125%. From graph correction factor = 1.00 (no effect)
21 Anion Design Anion Capacity Adjustment We can operate to a 200 ppb endpoint. From graph correction factor = 1.00 (No effect) We will have a bed depth above 0.7 m. From graph correction factor = 1.00 (No effect)
22 Anion Design Capacity (Theoretical) Silica percentage equates to 3.1% of Anion load. If we assume regenerant temperature of 10 C then from graph correction factor = If we now apply all these correction factors to the base capacity we will obtain the theoretical working capacity (Ignoring those which are 1.0 as they have no effect). Theoretical Working capacity = 37.5 g/l x 0.95 x = g/l as CaCO 3
23 Anion Design Rinse Correction Now I need to correct the capacity for the loading on to the bed caused when the resin is rinsed after regeneration with decationised water. For a co-flow regenerated anion resin I would use 6 BV final rinse (when new). Therefore the rinse correction in g/l as CaCO 3 is: 6 (bed volumes) x 160 (mg/l anion load) / 1000 = 0.96 g/l Therefore revised working capacity is now = g/l Depending on the design / actual knowledge of the water, and the engineering system being used, a smart engineer will now apply a design margin to ensure the resin manufacturers performance can be guaranteed for an operating plant for the warranty period.
24 Anion Design Design Margin / Working Capacity The selection of and the amount of design margin is critical to a well designed reliable plant. When I am doing these calculations I favour taking a larger design margin on the anion resin over the cation resin. This is because I want the plant to be cation limiting making conductivity control of the plant on exhaustion easier and also because anion resin performance usually falls off at a quicker rate than cation performance. I therefore tend to take a 10 to 15% design margin on the anion capacity and correspondingly lower 5 to 10% on the cation resin. Many engineers just take 10% design margin on both to make the plant more competitively priced etc. On this example I will apply 10% to both. Therefore anion working capacity = x 0.9 = g/l.
25 Anion Design Resin Volume If you recall we calculated back on slide 17 we calculated the anion load as Kg as CaCO 3 The resin volume required (in litres) is therefore the ionic load / the working capacity of the resin x 1000: ( / 30.07) / 1000 = 2665 litres We normally round up to nearest 25 litre bag quantity hence: 2675 litres required Anion regen level was 60 g/l. Therefore caustic applied per regen is 60 x 2675 / 1000 = Kg as 100% NaOH.
26 Cation Design Design Analysis and Water Production The cation load based on the original raw water will now be: Cations Calcium (Ca) 100 Magnesium (Mg) 50 Sodium (Na) 90 Potassium (K) 10 Cation Load = 250 mg/l as CaCO 3 The volume of water treated per cycle would be: (8 hours x 60 m) + Anion regen water + MB regen water (21 m 3 ) (Note: a co-flow anion normally uses between 10 and 12 BV of water per regen.) We will use 12 BV for the calculation, so with 2675 litres of resin per anion unit, it needs 12 x 2700 litres = litres (32.1 m 3 )
27 Cation Design - Cationic Load Therefore cation volume of treated water = (8 x 60) + 32 m m 3 = 533 m 3 The cation load per cycle is therefore 533 m 3 x 250 mg/l / 1000 = Kg as CaCO 3 Now we have the load per cycle to calculate the resin volume we need to now calculate from the resin manufacturers data the working capacity of the resin. For the basis of this calculation, I am basing on an 8% DVB cross linked, gel, polystyrenic, standard grade strong acid cation resin with a high capacity. Similar product available from all the leading suppliers.
28 Cation Design - Capacity Correction Factors For this type of resin the working capacity is determined from a base capacity dependent on regen level (amount of acid applied). This capacity then has various correction factors applied dependent on the following: The percentage bicarbonate present in influent water. The temperature of the water treated. The percentage sodium in the cation load. The kinetic loading. This will not apply as this is mainly linked to high TDS waters or high BV/h flow rates.
29 Cation Design Base Resin Capacity For this co-flow regenerated design I have chosen a regeneration level of 66 g/l HCl. This corresponds from the cation engineering bulletin to a base working capacity of 1.14 eq/l. This is 57 g/l as CaCO 3 (1.14 x 50)
30 Cation Design Cation Capacity Adjustment Bicarbonate percentage in Cation load 100 mg/l / 250 mg/l x 100 = 40%. From graph correction factor = 0.97 Design water temperature 10 C (minimum water temp) From graph correction factor = 0.96
31 Cation Design- Cation Capacity Adjustment Sodium percentage in cation load 100 mg/l / 250 mg/l x 100 = 40%. From graph correction factor = This graph applies to high TDS or high BV/h flow rates. In this design it does not apply as we are to the LHS of the graph where the factor is 1.0 (No effect).
32 Cation Design Working Capacity If we now apply all these correction factors to the base capacity we will obtain the theoretical working capacity (Ignoring those which are 1.0 as they have no effect). Theoretical Working capacity = 57 g/l x 0.97 x 0.96 x = If we now apply rinse correction for co-flow cation. I tend to use 5 BV for this calculation for a co-flow regenerated cation. = 5 BV x 250 mg/l cation load / 1000 = 1.25 g/l as CaCO 3 This gives a theoretical capacity of = g/l as CaCO 3 If we then apply 10% design margin we have a working capacity of: x 0.9 = g/l as CaCO 3
33 Cation Design Resin Volume If you recall we calculated back on slide 27 we calculated the cation load as Kg as CaCO 3 The resin volume required (in litres) is therefore the ionic load / the working capacity of the resin x 1000: ( / 47.83) / 1000 = 2785 litres We normally round up to nearest 25 litre bag quantity hence: 2800 litres required Cation regen level was 66 g/l. Therefore acid applied per regen is 66 x 2800 / 1000 = Kg as 100% HCl.
34 Checking for Neutral Effluent (Taken from earlier slides) Cation load = Kg as CaCO 3 Anion load = Kg as CaCO 3 Cation acid applied = Kg as 100% HCl Caustic applied = Kg as 100% NaOH If we convert the chemicals applied to as CaCO 3 we can establish the excess acid and caustic generated from a regeneration (Conversion factor for HCl is x 1.37 and for NaOH it is x 1.25). Acid applied = x 1.37 = Kg. Therefore excess acid is the = Kg as CaCO 3 Caustic applied = x 1.25 = Kg. Therefore excess acid is the = Kg as CaCO 3 The two excesses are similar therefore neutral effluent!
35 Checking for Neutral Effluent I CHEATED I did the calculation first before preparing the slides and this is why I selected a cation regen level of 66 g/l. I knew it gave me a neutral effluent for the calculation/presentation Otherwise this part of the hand calculation takes some time to resolve. You have to draw a graph on which you plot regeneration level against excess regenerant generated and regeneration level against resin volume. Based on this graph it is then possible to interpret the results to reach a neutral effluent but it takes some time! Probably the subject of a another presentation. THIS IS WHERE DESIGN PROGRAMMES HELP SO MUCH
36 Vessel Sizing Design Parameters Fortunately in this example co-flow cation and anion have very similar resin volumes so the sizing will be almost identical (2.675 m 3 in the anion and m 3 in the cation. For insitu regenerated co-flow regenerated plant I use the following parameters for my vessel sizing: Maximum service velocity. 50m 3 /m 2 /h (m/h) Minimum service velocity. 12m 3 /m 2 /h (m/h) Guide to maximum pressure drop at minimum temperature for a fully classified bed to allow for some compaction/fouling. 100 to 122 Kpa. Maximise bed depth within pressure drop guide but rarely would the bed depth exceed 1.75 m and preferably more than 1.0 m and never below 0.6 m. 50 to 60% freeboard above resin for co-flow backwash.
37 Vessel Sizing Using these parameters and the pressure drop curves for each resin the vessel size I would have selected the same vessel size for both columns: Metric 1600 mm diameter x 2250 mm i/s. This corresponds to 1.40 m cation and 1.34 m anion bed depth (installed) and a service velocity of 30 m 3 /m 2 /h (m/h) UK 5 feet diameter x 8.25 feet i/s This corresponds to 1.54 m cation and 1.47 m anion bed depth (installed) and a service velocity of 33 m 3 /m 2 /h (m/h)
38 Pressure Drop Across Resin Beds We know the bed depths 1.4 m (cation) and 1.34 m (anion) and because the vessels are the same diameter the velocity of the water through each bed is the same (30 m 3 /m 2 /h (m/h)). If we assume the minimum water temperature is 10 C in the winter, we can now calculate the pressure drop from a single graph for each resin which is based on the. Velocity Water Temperature Bed Depth
39 Pressure Drop Across Resin Beds Anion Resin Example Different graph for cation resin At a velocity of 30 m/h and temperature of 10 C (green line) we can read of the pressure drop. In this case the answer is 46 kpa/m. Our bed depth is 1.34 m so pressure drop across a clean, fully classified, not compacted bed is 46 x 1.34 = Kpa. For my pump calculations I add a safety margin between 10 and 20% depending on how free of solids the water is, cycle length (compaction), resin ageing etc. Therefore pressure loss for pump is around 70 Kpa within limit.
40 Leakage from Resin Beds Cation Resin Example From the cation regen level selected, and the raw water analysis we can calculate the sodium leakage from the cation resin which allows us to establish the conductivity exit the anion. Reactive silica leakage from anion makes no contribution to the anion outlet conductivity. Similar to the capacity calculation the leakage starts with the base leakage based on the regeneration level. Then we apply factors. In this case the factors are: 1. The EMA present in the feed in meq/l. (EMA = Sulphate + Nitrate + Chloride) 2. The % Sodium in the feed as CaCO3.
41 Leakage from Resin Beds Cation Resin Example Regen level selected 66g/l. From slide 9: Sodium % in feed 90/250 = 36% EMA level in meq/l 150 mg/l / 50 = 3.0 Sodium Leakage 7 mg/l x 0.35 x 0.75 = 1.83 mg/l Therefore average leakage 2 mg/l as Na
42 Leakage from Resin Beds Anion Resin From the anion regen level selected, and the raw water analysis we can calculate the reactive silica leakage from the anion resin. Similar to the cation calculation the leakage starts with the base leakage based on the regeneration level. Then we apply factors. In this case there are five factors which are: 1. The % Reactive Silica to Total Anions. 2. Feed Water Temperature (Highest temp gives highest leakage correction). 3. Regenerant Temperature (Highest temp gives lowest leakage correction). 4. Sodium leakage from cation in mg/l Na. 5. Silica end point.
43
Ion Exchange RESIN SELECTION. Marc Slagt Technical Support Specialist DOW Water & Process Solutions
Ion Exchange RESIN SELECTION Marc Slagt Technical Support Specialist DOW Water & Process Solutions I WILL BRING YOU HAPPINESS!! Resin selection = HAPPINESS Why happiness... When it comes to resin selection
More informationResin Types and Production. Brian Windsor (Purolite International Ltd)
Resin Types and Production Brian Windsor (Purolite International Ltd) Properties of an Ion Exchanger Synthetic Ion Exchangers require certain properties to perform demineralisation. The three main properties
More informationC-100E Strong Acid Cation Exchange Resin (For use in water softening applications)
ION EXCHANGE RESINS C-1E Strong Acid Cation Exchange Resin (For use in water softening applications) Lenntech info@lenntech.com www.lenntech.com Tel. +31-15-61.9. Fax. +31-15-61.6.89 Technical Data PRODUCT
More informationION EXCHANGE FOR DUMMIES. An introduction
ION EXCHANGE FOR DUMMIES An introduction Water Water is a liquid. Water is made of water molecules (formula H 2 O). All natural waters contain some foreign substances, usually in small amounts. The water
More informationDEIONIZATION IN A 'NUT SHELL'
Deionized Water (DI) DEIONIZATION IN A 'NUT SHELL' City water is passed through dark amber colored, caviar sized plastic beads called cation ion exchange resin. The cation resin is in the hydrogen form
More informationLewatit MP 62 is especially suitable for:
Lewatit MP 62 is a weakly basic, macroporous anion exchange resin with tertiary amine groups (monofunctional), hence of particularly low basicity, and of standard bead size distribution. Its high total
More informationGUIDELINES FOR SELECTING RESIN ION EXCHANGE OR REVERSE OSMOSIS FOR FEED WATER DEMINERALISATION
GUIDELINES FOR SELECTING RESIN ION EXCHANGE OR REVERSE OSMOSIS FOR FEED WATER DEMINERALISATION Prepared by: Purolite International Date: November 2003 Operating Puropack Plant 2 GUIDELINES FOR SELECTING
More informationDow Liquid Separations DOWEX SBR-P. Ion Exchange Resin ENGINEERING INFORMATION
Dow Liquid Separations DOWEX SBR-P Ion Exchange Resin ENGINEERING INFORMATION DOWEX SBR-P Type 1 Strong Base Anion Exchange Resin General Information DOWEX* SBR-P resin is a gel type 1 strong base anion
More informationDow Liquid Separations DOWEX MARATHON A. Ion Exchange Resin ENGINEERING INFORMATION
Dow Liquid Separations DOWEX MARATHON A Ion Exchange Resin ENGINEERING INFORMATION DOWEX MARATHON A Type 1 Strong Base Anion Exchange Resin General Information DOWEX* MARATHON* A resin is a high capacity,
 More information
More information Dow Liquid Separations DOWEX MARATHON MSA. Ion Exchange Resin. Engineering Information
Dow DOWEX MARATHON MSA Ion Exchange Resin Engineering Information DOWEX MARATHON MSA Macroporous Type I Strong Base Anion Exchange Resin General Information DOWEX MARATHON MSA is a high capacity, macroporous,
More informationCulligan Exchange Tank Deionization Service
Culligan Exchange Tank Deionization Service Beverages Boiler Feedwater Distilleries Food Preparation/Processing Glass/Mirrors Humidification Ice Making Photo Processing Plating/Anodizing Printing Vehicle
More informationION EXCHANGE RESINS INTRODUCTION
ION EXANGE RESINS Ion exchange resins are polymers that are capable of exchanging particular ions within the polymer with ions in a solution that is passed through them. This ability is also seen in various
More informationLewatit MonoPlus SP 112 H is especially suitable for:
Lewatit MonoPlus SP 112 H is a strongly acidic, macroporous cation exchange resin with beads of uniform size (monodisperse) based on a styrene-divinylbenzene copolymer, in fully regenerated form (min.
More informationPurolite Water Softening Resin Guide By: Chubb Michaud
Application Notes Water Softening Basics Purolite Water Softening Resin Guide By: Chubb Michaud Water, passing through the atmosphere as snow or rain, picks up carbon dioxide (CO2) and other acid gases
More informationInferred ph in Steam Plant Water Chemistry Monitoring
Application Data Sheet ADS 4900-87/rev.B January 2009 Power Industry Inferred ph in Steam Plant Water Chemistry Monitoring INTRODUCTION Inferred ph means ph calculated from straight and cation conductivity.
More informationSODIUM CATION EXCHANGE (ZEOLITE) WATER SOFTENING PROCESS
SODIUM CATION EXCHANGE (ZEOLITE) WATER SOFTENING PROCESS A. History The name zeolite comes from the two Greek words zein and lithos which mean boiling stone. It was first applied by Granstedt, a Swedish
More informationAPPLICATION GUIDE Choosing an ion exchange system for nitrate removal
APPLICATION GUIDE Choosing an ion exchange system for nitrate removal Nitrate levels are coming under stricter control due to health and environmental concerns. Ion exchange technology not only effectively
More informationATOMS. Multiple Choice Questions
Chapter 3 ATOMS AND MOLECULES Multiple Choice Questions 1. Which of the following correctly represents 360 g of water? (i) 2 moles of H 2 0 (ii) 20 moles of water (iii) 6.022 10 23 molecules of water (iv)
More informationIon Exchange Softening
Ion Exchange Softening Ion-exchange is used extensively in small water systems and individual homes. Ion-exchange resin, (zeolite) exchanges one ion from the water being treated for another ion that is
More informationISR IRON REMOVAL MEDIA. Characteristics. Recommended influent Conditions. Description
ISR IRON REMOVAL MEDIA Description INDION ISR is a special media designed to provide excellent catalytic properties to remove dissolved iron from ground water. INDION ISR is an insoluble media which oxidizes
More informationWater Softening for Hardness Removal. Hardness in Water. Methods of Removing Hardness 5/1/15. WTRG18 Water Softening and Hardness
Water Softening for Removal 1 in Water High concentration of calcium (Ca2+) and magnesium (Mg2+) ions in water cause hardness Generally, water containing more than 100 mg/l of hardness expressed as calcium
More informationGCSE Chemistry. Making Salts Instructions and answers for teachers
GCSE Chemistry Making Salts Instructions and answers for teachers The Activity: Learning Outcomes: To be able to recall the names and chemical formulae for commonly used acids To understand how salts can
More informationExperiment 8 - Double Displacement Reactions
Experiment 8 - Double Displacement Reactions A double displacement reaction involves two ionic compounds that are dissolved in water. In a double displacement reaction, it appears as though the ions are
More informationIon Exchange Resins for Metal Plating and Surface Finishing
Ion Exchange Resins for Metal Plating and Surface Finishing ION EXCHANGE RESINS Contents Introduction 5 Plating Bath Rejuvenation 6 Rinse Water Recycling 8 Waste Effluent Treatment 12 Copper Recovery 16
More informationThe Relationship between ph and Deionized Water
The Relationship between ph and Deionized Water The basics of ph The topic of ph and water has been well documented over the years; however, there is still much confusion about its significance in high
More informationHardness - Multivalent metal ions which will form precipitates with soaps. e.g. Ca 2+ + (soap) Ca(soap) 2 (s)
Water Softening (Precipitation Softening) (3 rd DC 178; 4 th DC 235) 1. Introduction Hardness - Multivalent metal ions which will form precipitates with soaps. e.g. Ca 2+ + (soap) Ca(soap) 2 (s) Complexation
More informationDissolved and precipitated oxalate
Accepted 2005 Process liquors from bleach plants Dissolved and precipitated oxalate Using Ion Chromatography 0 Introduction In bleach plants of pulp mills with a high degree of system closure, there is
More information2. DECOMPOSITION REACTION ( A couple have a heated argument and break up )
TYPES OF CHEMICAL REACTIONS Most reactions can be classified into one of five categories by examining the types of reactants and products involved in the reaction. Knowing the types of reactions can help
More informationTechnology Selection Tools for Boiler Feedwater Applications (US Units)
Technical Paper Technology Selection Tools for Boiler Feedwater Applications (US Units) Authors: Robert Gerard and Roch Laflamme, GE Water & Process Technologies Introduction During the last decade, the
More informationTutorial 4 SOLUTION STOICHIOMETRY. Solution stoichiometry calculations involve chemical reactions taking place in solution.
T-27 Tutorial 4 SOLUTION STOICHIOMETRY Solution stoichiometry calculations involve chemical reactions taking place in solution. Of the various methods of expressing solution concentration the most convenient
More informationMajor Ions in Water. Training module # WQ - 28. New Delhi, September 1999
World Bank & Government of The Netherlands funded Training module # WQ - 28 Major Ions in Water New Delhi, September 1999 CSMRS Building, 4th Floor, Olof Palme Marg, Hauz Khas, New Delhi 11 00 16 India
More informationChem101: General Chemistry Lecture 9 Acids and Bases
: General Chemistry Lecture 9 Acids and Bases I. Introduction A. In chemistry, and particularly biochemistry, water is the most common solvent 1. In studying acids and bases we are going to see that water
More informationWastewater Reuse. Typical treated wastewater is:
Wastewater Reuse Most metal finishing industries have in-house wastewater treatment to economically dispose of the acids, alkali, oils, and dissolved metals in the rinse water and occasional tank solution
More information6 Reactions in Aqueous Solutions
6 Reactions in Aqueous Solutions Water is by far the most common medium in which chemical reactions occur naturally. It is not hard to see this: 70% of our body mass is water and about 70% of the surface
More informationThis document contains important information and must be read in its entirety. Edition: 2011-10-13 Previous Edition: 2011-05-12 1/4
Lewatit MonoPlus TP 214 is a monospherical, macroporous chelating resin with thiourea groups having a high affinity for mercury. Next to this special mercury selectivity, Lewatit MonoPlus TP 214 has a
More informationWRITING CHEMICAL FORMULA
WRITING CHEMICAL FORMULA For ionic compounds, the chemical formula must be worked out. You will no longer have the list of ions in the exam (like at GCSE). Instead you must learn some and work out others.
More informationIndustrial Water Reuse and Wastewater Minimization
Technical Paper Industrial Water Reuse and Wastewater Minimization Author: James P. McIntyre, P.E. Abstract Many industrial users of fresh water are under increasing pressure to reuse water within their
More informationTHEORY AND APPLICATION OF CONDUCTIVITY
Application Data Sheet ADS 43-018/rev.D January 2010 Theory THEORY AND APPLICATION OF CONDUCTIVITY BACKGROUND Conductivity is a measure of how well a solution conducts electricity. To carry a a solution
More informationHardness ions also interfere with many chemical processes such as chemical compounding and aqueous cleaners.
Water Softeners Industrial Water Purification (800) CAL-WATER By Dave Peairs, Cal Water, Technical Director Rev: 06/08/2004 Before any discussion of water softeners, we must first define what hard water
More informationRemoving Thallium from Industrial FGD Scrubber Water with Sorbster Adsorbent Media
Case History MAR Systems Inc. Removing Thallium from Industrial FGD Scrubber Water with Sorbster Adsorbent Media Trace thallium levels in process and wastewater streams pose a human toxicity threat. Tidwell
More informationProperties of Acids and Bases
Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? What
More informationChapter 17. How are acids different from bases? Acid Physical properties. Base. Explaining the difference in properties of acids and bases
Chapter 17 Acids and Bases How are acids different from bases? Acid Physical properties Base Physical properties Tastes sour Tastes bitter Feels slippery or slimy Chemical properties Chemical properties
More informationCHEMICAL PRECIPITATION: WATER SOFTENING
CHEMICAL PRECIPITATION: WATER SOFTENING Submitted to: Dr. Hashsham Research Complex Engineering Department of Civil and Environmental Engineering Michigan State University East Lansing, MI 4884 Authors
More informationTHE USE OF OZONE IN COOLING TOWERS
THE USE OF OZONE IN COOLING TOWERS Paul D. McNicholas Ozonia Ltd Duebendorf, Switzerland Abstract Ozone has been successfully applied to industrial cooling water systems with the resultant improvement
More informationIon Exchange Design Calculation
EXTRACTION OF METALS
1 EXTRACTION OF METALS Occurrence ores of some metals are very common (iron, aluminium) others occur only in limited quantities in selected areas ores need to be purified before being reduced to the metal
More informationQuestion Bank Electrolysis
Question Bank Electrolysis 1. (a) What do you understand by the terms (i) electrolytes (ii) non-electrolytes? (b) Arrange electrolytes and non-electrolytes from the following substances (i) sugar solution
More informationNumber of moles of solute = Concentration (mol. L ) x Volume of solution (litres) or n = C x V
44 CALCULATIONS INVOLVING SOLUTIONS INTRODUCTION AND DEFINITIONS Many chemical reactions take place in aqueous (water) solution. Quantities of such solutions are measured as volumes, while the amounts
More informationImproving Silica Removal By EDI and GTM January 16, 2014 August 27, 2014 -- McIlvaine
Improving Silica Removal By EDI and GTM January 16, 2014 August 27, 2014 McIlvaine Michael J. Snow, Ph.D. President SnowPure Water Technologies (USA) San Clemente, California Headquarters Electropure EDI
More informationDOWEX Resins as Organic Solvent Desiccants
Product Information DOWEX Resins as Organic Solvent Desiccants DOWEX* ion exchange resins can be used as desiccants for organic solvents, after having been dried to a low moisture level, in a manner similar
More information1. Inspection and monitoring... 3
1 Index 1. Inspection and monitoring... 3 1.1 Handling of new elements... 3 1.1.1 Storage of original packaged RO elements... 3 1.1.2 Packing... 3 1.2 Initial start- up checks of a plant... 3 1.2.1 Preparation
More informationChapter 16: Tests for ions and gases
The position of hydrogen in the reactivity series Hydrogen, although not a metal, is included in the reactivity series because it, like metals, can be displaced from aqueous solution, only this time the
More informationTopic 4 National Chemistry Summary Notes. Formulae, Equations, Balancing Equations and The Mole
Topic 4 National Chemistry Summary Notes Formulae, Equations, Balancing Equations and The Mole LI 1 The chemical formula of a covalent molecular compound tells us the number of atoms of each element present
More informationCHEMICAL REACTIONS AND REACTING MASSES AND VOLUMES
CHEMICAL REACTIONS AND REACTING MASSES AND VOLUMES The meaning of stoichiometric coefficients: 2 H 2 (g) + O 2 (g) 2 H 2 O(l) number of reacting particles 2 molecules of hydrogen react with 1 molecule
More informationDetermination of the amount of sodium carbonate and sodium hydroxide in a mixture by titration.
Module 9 : Experiments in Chemistry Lecture 38 : Titrations : Acid-Base, Redox and Complexometric Objectives In this lecture you will learn the techniques to do following Determination of the amount of
More informationUPCORE System. Suspended Solids Removal for Countercurrent Ion Exchange Systems
UPCORE System Suspended Solids Removal for Countercurrent Ion Exchange Systems Daniel B. Rice - The Dow Chemical Company, Midland, Michigan Andre Medete - Dow Deutschland Inc., Rheinmuenster, Germany Published
More informationChemistry 132 NT. Solubility Equilibria. The most difficult thing to understand is the income tax. Solubility and Complex-ion Equilibria
Chemistry 13 NT The most difficult thing to understand is the income tax. Albert Einstein 1 Chem 13 NT Solubility and Complex-ion Equilibria Module 1 Solubility Equilibria The Solubility Product Constant
More informationq = 6.74x1 =6.74 10-1 mg/l x 3.78x10 L/d = 3.4x10 mg / day a) Single CMFR mg/g C Organic Load = Carbon requirement = 6.74 mg 1000 g C inf = 10 mg/l
Example An industrial wastewater contains 10 mg/l chlorophenol, and is going to be treated by carbon adsorption. 90% removal is desired. The wastewater is discharged at a rate of 0.1 MGD. Calculate the
More informationFactors Affecting Precipitation of Calcium Carbonate
Factors Affecting Precipitation of Calcium Carbonate John A. Wojtowicz Chemcon Laboratory tests with clear solutions showed that precipitation of calcium carbonate does not occur in the ph range 7.5 to
More informationENE 806, Project Report 3 CHEMICAL PRECIPITATION: WATER SOFTENING. Grégoire Seyrig Wenqian Shan
ENE 806, Project Report 3 CHEMICAL PRECIPITATION: WATER SOFTENING Grégoire Seyrig Wenqian Shan College of Engineering, Michigan State University Spring 2007 ABSTRACT The groundwater with high level initial
More informationBalancing Chemical Equations Worksheet
Balancing Chemical Equations Worksheet Student Instructions 1. Identify the reactants and products and write a word equation. 2. Write the correct chemical formula for each of the reactants and the products.
More information1. Read P. 368-375, P. 382-387 & P. 429-436; P. 375 # 1-11 & P. 389 # 1,7,9,12,15; P. 436 #1, 7, 8, 11
SCH3U- R.H.KING ACADEMY SOLUTION & ACID/BASE WORKSHEET Name: The importance of water - MAKING CONNECTION READING 1. Read P. 368-375, P. 382-387 & P. 429-436; P. 375 # 1-11 & P. 389 # 1,7,9,12,15; P. 436
More informationPurolite Ion Exchange Design Calculation Program
DETERMINING THE ENTHALPY OF FORMATION OF CaCO 3
DETERMINING THE ENTHALPY OF FORMATION OF CaCO 3 Standard Enthalpy Change Standard Enthalpy Change for a reaction, symbolized as H 0 298, is defined as The enthalpy change when the molar quantities of reactants
More informationExperiment 16-Acids, Bases and ph
Definitions acid-an ionic compound that releases or reacts with water to form hydrogen ion (H + ) in aqueous solution. They taste sour and turn litmus red. Acids react with certain metals such as zinc,
More informationWATER CHEMISTRY AND POOL WATER BALANCE
C R6 H A PT E WATER CHEMISTRY AND POOL WATER BALANCE LEARNING OBJECTIVES After completely studying this chapter, you should be able to: Understand and list the parameters upon which water balance is based.
More information20.2 Chemical Equations
All of the chemical changes you observed in the last Investigation were the result of chemical reactions. A chemical reaction involves a rearrangement of atoms in one or more reactants to form one or more
More informationBalancing Chemical Equations
Balancing Chemical Equations Academic Success Center Science Tutoring Area Science Tutoring Area Law of Conservation of Mass Matter cannot be created nor destroyed Therefore the number of each type of
More informationEquilibria Involving Acids & Bases
Week 9 Equilibria Involving Acids & Bases Acidic and basic solutions Self-ionisation of water Through reaction with itself: The concentration of water in aqueous solutions is virtually constant at about
More informationFormulae, stoichiometry and the mole concept
3 Formulae, stoichiometry and the mole concept Content 3.1 Symbols, Formulae and Chemical equations 3.2 Concept of Relative Mass 3.3 Mole Concept and Stoichiometry Learning Outcomes Candidates should be
More informationTREATMENT OF PHOSPHATE FERTILIZER PLANT WASTE WATER IN FLORIDA FOR DISCHARGE AND RE USE PURPOSES
TREATMENT OF PHOSPHATE FERTILIZER PLANT WASTE WATER IN FLORIDA FOR DISCHARGE AND RE USE PURPOSES JOHN F. BOSSLER, SIEMENS Water Technologies Corp., Hoffman Estates, IL RONALD TRAVIS, SIEMENS Water Technologies
More informationMolarity of Ions in Solution
APPENDIX A Molarity of Ions in Solution ften it is necessary to calculate not only the concentration (in molarity) of a compound in aqueous solution but also the concentration of each ion in aqueous solution.
 More information
More information Continuous process of sodium bicarbonate production by Solvay method
Continuous process of sodium bicarbonate production by Solvay method Manual to experiment nr 10 Instructor: Dr Tomasz S. Pawłowski 1 Goal of the experiment The goal of the experiment is introduction of
More informationCHEMICAL DETERMINATION OF EVERYDAY HOUSEHOLD CHEMICALS
CHEMICAL DETERMINATION OF EVERYDAY HOUSEHOLD CHEMICALS Purpose: It is important for chemists to be able to determine the composition of unknown chemicals. This can often be done by way of chemical tests.
More informationA meaningful, cost-effective solution for polishing reverse osmosis permeate
A meaningful, cost-effective solution for polishing reverse osmosis permeate Electrodeionization or EDI, is a continuous and chemicalfree process of removing ionized and ionizable species from the feed
More informationFILMTEC. Membranes. The Economics of Reverse Osmosis and Ion Exchange. Dow Liquid Separations. WATERTECH Expo '94 November 9-11, 1994 Houston, Texas
Dow Liquid Separations FILMTEC Membranes The Economics of Reverse Osmosis and Ion Exchange WATERTECH Expo '94 November 9-11, 1994 Houston, Texas by: Scott S. Beardsley - The Dow Chemical Company Steven
More information(1) e.g. H hydrogen that has lost 1 electron c. anion - negatively charged atoms that gain electrons 16-2. (1) e.g. HCO 3 bicarbonate anion
GS106 Chemical Bonds and Chemistry of Water c:wou:gs106:sp2002:chem.wpd I. Introduction A. Hierarchy of chemical substances 1. atoms of elements - smallest particles of matter with unique physical and
More informationQ.1 Classify the following according to Lewis theory and Brønsted-Lowry theory.
Acid-base 2816 1 Acid-base theories ACIDS & BASES - IONIC EQUILIBRIA LEWIS acid electron pair acceptor H +, AlCl 3 base electron pair donor NH 3, H 2 O, C 2 H 5 OH, OH e.g. H 3 N: -> BF 3 > H 3 N + BF
More informationTechnical Presentation IMPORTANT TOPICS
BOILER WATER TREATMENT FOR KILN DRY OPERATIONS Technical Presentation IMPORTANT TOPICS PRETREATMENT TEMPERATURE VS OXYGEN FEED WATER / DA BOILER WATER CONDENSATE 1 Boiler Water Pretreatment Purpose - Statistically
More informationACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND
#3. Acid - Base Titrations 27 EXPERIMENT 3. ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND Carbonate Equilibria In this experiment a solution of hydrochloric
More informationEXPERIMENT 2 THE HYDROLYSIS OF t-butyl CHLORIDE. PURPOSE: To verify a proposed mechanism for the hydrolysis of t-butyl Chloride.
PURPOSE: To verify a proposed mechanism for the hydrolysis of t-butyl Chloride. PRINCIPLES: Once the Rate Law for a reaction has been experimentally established the next step is its explanation in terms
More information1A Rate of reaction. AS Chemistry introduced the qualitative aspects of rates of reaction. These include:
1A Rate of reaction AS Chemistry introduced the qualitative aspects of rates of reaction. These include: Collision theory Effect of temperature Effect of concentration Effect of pressure Activation energy
More informationWell Water Iron Removal Using Quantum DMI-65 Granular Filter Media
Well Water Iron Removal Using Quantum DMI-65 Granular Filter Media ASME Research Committee Power Plant and Environmental Chemistry Overland Park, Kansas April 2-4, 2007 Prepared by: W. H. Stroman Primary
More informationQUESTION (2012:3) (a) (i) Complete the table below showing the conjugate acids and bases. CO 3 H 2 O OH HCN CN -
QUESTION (2012:3) (i) Complete the table below showing the conjugate acids and bases. Conjugate acid Conjugate base - HCO 3 2 CO 3 H 2 O OH HCN CN - (ii) HPO 4 2 (aq) Write equations for the reactions
More informationChemistry Post-Enrolment Worksheet
Name: Chemistry Post-Enrolment Worksheet The purpose of this worksheet is to get you to recap some of the fundamental concepts that you studied at GCSE and introduce some of the concepts that will be part
More informationLAB 5 - PLANT NUTRITION. Chemical Ionic forms Approximate dry Element symbol Atomic weight Absorbed by plants tissue concentration
LAB 5 PLANT NUTRITION I. General Introduction All living organisms require certain elements for their survival. Plants are known to require carbon (C), hydrogen (H), oxygen (O), nitrogen (N), phosphorus
More informationSyllabus OC18 Use litmus or a universal indicator to test a variety of solutions, and classify these as acidic, basic or neutral
Chemistry: 9. Acids and Bases Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC18 Use litmus or a universal indicator to test a variety
More informationChapter 8: Chemical Equations and Reactions
Chapter 8: Chemical Equations and Reactions I. Describing Chemical Reactions A. A chemical reaction is the process by which one or more substances are changed into one or more different substances. A chemical
More information7.4. Using the Bohr Theory KNOW? Using the Bohr Theory to Describe Atoms and Ions
7.4 Using the Bohr Theory LEARNING TIP Models such as Figures 1 to 4, on pages 218 and 219, help you visualize scientific explanations. As you examine Figures 1 to 4, look back and forth between the diagrams
More informationph Alkalinity of Water
ph Alkalinity of Water DOC316.52.93085 Based on ISO standard 9963-1:1994 ph-metric Titration 0.4 to 20 mmol/l of Total Alkalinity 1. Introduction Alkalinity of water is its acid-neutralizing capacity.
More informationChemistry: Chemical Equations
Chemistry: Chemical Equations Write a balanced chemical equation for each word equation. Include the phase of each substance in the equation. Classify the reaction as synthesis, decomposition, single replacement,
 More information
More information Removing Heavy Metals from Wastewater
Removing Heavy Metals from Wastewater Engineering Research Center Report David M. Ayres Allen P. Davis Paul M. Gietka August 1994 1 2 Removing Heavy Metals From Wastewater Introduction This manual provides
More informationEstimation of Hardness of Water by EDTA Method
Estimation of Hardness of Water by EDTA Method 1 EXPERIMENT 1 Estimation of Hardness of Water by EDTA Method INTRODUCTION Water hardness is the traditional measure of the capacity of water to precipitate
More informationDescription of the Mole Concept:
Description of the Mole Concept: Suppose you were sent into the store to buy 36 eggs. When you picked them up you would get 3 boxes, each containing 12 eggs. You just used a mathematical device, called
More informationQ.1 Classify the following according to Lewis theory and Brønsted-Lowry theory.
Acid-base A4 1 Acid-base theories ACIDS & BASES - IONIC EQUILIBRIA 1. LEWIS acid electron pair acceptor H, AlCl 3 base electron pair donor NH 3, H 2 O, C 2 H 5 OH, OH e.g. H 3 N: -> BF 3 > H 3 N BF 3 see
More informationTo determine the equivalence points of two titrations from plots of ph versus ml of titrant added.
Titration Curves PURPOSE To determine the equivalence points of two titrations from plots of ph versus ml of titrant added. GOALS 1 To gain experience performing acid-base titrations with a ph meter. 2
More informationBest Practice in Boiler Water Treatment
Best Practice in Boiler Water Treatment Boiler Water Treatment Part 2 Internal Treatment Objectives of Internal Water Treatment 1 To control the level of total dissolved solids (TDS) within the boiler
More informationQ1. A student studied the reaction between dilute hydrochloric acid and an excess of calcium carbonate.
Q. A student studied the reaction between dilute hydrochloric acid and an excess of calcium carbonate. calcium carbonate + hydrochloric acid calcium chloride + water + carbon dioxide The student measured
More informationDEPARTMENT OF ENVIRONMENTAL REGULATION. Technical Document DETERMINING REPRESENTATIVE GROUND WATER SAMPLES, FILTERED OR UNFILTERED
DEPARTMENT OF ENVIRONMENTAL REGULATION Technical Document DETERMINING REPRESENTATIVE GROUND WATER SAMPLES, FILTERED OR UNFILTERED JANUARY 1994 BUREAU OF DRINKING WATER AND GROUND WATER RESOURCES 2600 BLAIR
More informationNotes Chapter 9 Limiting Reagent Sample Problems Page 1
Notes Chapter 9 Limiting Reagent Sample Problems Page 1 Problem 1: Sodium chloride can be prepared by the reaction of sodium metal with chlorine gas. Suppose that 6.70 Na reacts with 3.20 Cl 2. A. What
More informationRemediation of VOC Contaminated Groundwater
Remediation of VOC Contaminated Groundwater Background Contaminated groundwater has become an ever-increasing problem in the United States and around the world. Treatment and disposal of waste is a serious
More informationCalculation of Molar Masses. Molar Mass. Solutions. Solutions
Molar Mass Molar mass = Mass in grams of one mole of any element, numerically equal to its atomic weight Molar mass of molecules can be determined from the chemical formula and molar masses of elements
More information